COVID Transmissions for 10-27-2021
FDA evaluation of vaccination of children for COVID-19 by Pfizer's COMIRNATY
Greetings from an undisclosed location in my apartment. Welcome to COVID Transmissions.
It has been 710 days since the first documented human case of COVID-19. In 710, Pope Constantine visited Constantinople, the Roman (Byzantine) capital named for Emperor Constantine. Pope Constantine becomes the last of his office to visit that city for more than a thousand years, and certainly the last to visit while it still bore the name Constantinople.1
Today’s issue is dedicated to Pfizer data that was used by the FDA to evaluate the vaccination of children ages 5 to 11 with a lower-dose formulation of that company’s vaccine. There’s a lot to dig into.
Bolded terms are linked to the running newsletter glossary.
Keep COVID Transmissions growing by sharing it! Share the newsletter, not the virus. I love talking about science and explaining important concepts in human health, but I rely on all of you to grow the audience for this, which you can do by using this button here:
Now, let’s talk COVID.
Pfizer’s Comirnaty is on the way to Emergency Use Authorization in 5-11 year old children in the US
Yesterday, the FDA’s VRBPAC2 voted 19-0 with 1 abstention in favor of expanding the Pfizer vaccine’s Emergency Use Authorization (EUA) to cover children 5-11 years old, using a special formulation of the vaccine that has 1/3 the mRNA dosage compared with what is used in patients 12 years and older. CNN summarizes some of the meeting here: https://www.cnn.com/2021/10/26/health/covid-19-young-kids-vaccine-fda-discussion/index.html
Yesterday’s news about this comes at great relief to many parents, as I understand. I have been carefully avoiding this topic until today, because I wanted to provide a more comprehensive update today instead of little news bites about this all throughout the development of the story. We didn’t have a lot of data until now, and I didn’t want to go off about this important story without a complete picture.
Pfizer released its topline results in this child age group about a month ago: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-positive-topline-results. These topline results were followed today with a more comprehensive briefing book that was used by the VRBPAC: https://www.fda.gov/media/153447/download.
In the vaccine studies for young children, Pfizer elected not to use a traditional measurement of vaccine efficacy, instead focusing on “immunobridging” as an endpoint. Readers, I had never heard the word immunobridging before I heard about the Pfizer clinical trials in children.
Immunobridging is the idea that we can use immunological data from a known efficacy population to set the goalposts for immunological data for a population where efficacy data are not being collected. In other words, Pfizer had efficacy data in a group of people ages 16-25, and also data on immune responses in those people. They proposed that if their product created similar immune responses in people ages 12-15 and 5-11 years compared with what they saw in 16-25, then the efficacy would be similar. Specifically, they focused on neutralizing antibody levels in their group of interest, compared with the older set, as a key endpoint for their study. This is not that different from using correlates of protection, except that at the time these studies were designed, there really wasn’t any information on correlates of protection from COVID-19. So Pfizer had to use this immunobridging concept. As it turns out, they made the right call, because it has since become clear that neutralizing antibody responses are indeed a correlate of protection from COVID-19.
That’s the what of their endpoint selection, but here’s the why. We know that COVID-19 has so far been less severe in children, in general. This is no comfort at all to those parents whose children have died of COVID-19, but it does complicate the design of clinical trials. Normally, a trialist would design a trial to reach a certain number of cases across the total trial population, and use that to compare placebo effects to vaccinated group effects. In this case, however, it was not clear how many cases could be expected. Because of an expectation of relatively milder disease, it’s really hard to say how many disease cases the trial population might have in this group. It might have taken an exceptionally long time to be able to accrue enough case events to be able to measure vaccine efficacy with enough statistical power.
Immunological data don’t have this unexpected follow-up time. We know how long it takes for immune responses to form; antibodies tend to be fully matured between 9 and 11 days after exposure to an antigen. This is why the definition of “fully vaccinated” is 14 days after your final dose of whatever vaccine regimen you have opted for; that ensures that your immune system will have had the chance to mature its response to the vaccine and thus protect you from exposure to SARS-CoV-2.
So, with an immunological endpoint rather than an efficacy endpoint, Pfizer could much more easily conduct their trial—without making people wait an inordinate amount of time during a global emergency. They would be able to predict that the trial would be finished about 8 weeks after they enrolled their last patient. Astute readers will notice this is not 6 weeks; that’s because they had some additional safety follow-up weeks. A prior review of vaccine safety events in clinical trials—conducted, actually, for the EUA of the adult version of this vaccine—determined that no vaccine has ever had a new safety event emerge more than 6 weeks after administration. With 8 weeks from dose 1, there is some margin of safety beyond that 6 week timing.
Additionally, patients were followed for detection of serious adverse events for up to 6 months or to the study’s data cutoff date3—whichever came first for that individual patient. This is another design feature intended to err on the side of caution.
These design characteristics all make the study more predictable in its timing, which as I mentioned is important when there is a worldwide population of parents who are highly concerned about their children being exposed to a dangerous pandemic illness.
Now, to the results. For the immunobridging endpoint, Pfizer used a ratio of neutralizing antibody levels in 5-11 year old patients to the levels in 16-25 year old patients. Please note that the 5-11 year olds received a 1/3 dose by comparison the older group. What Pfizer was looking for was a ratio that comes out to 1. They wanted the antibody titers to be approximately the same, despite the lower dose. The ratio they got? 1.04, with a 95% confidence interval of 0.93 to 1.18, as shown in this data table:
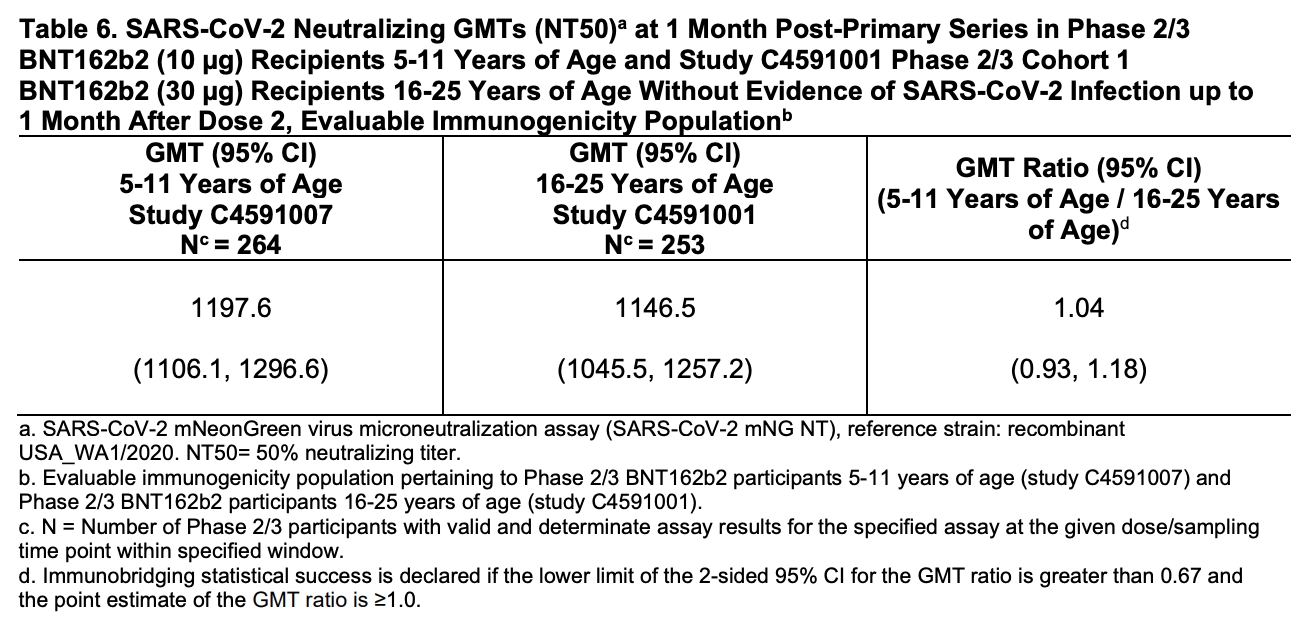
I’d say they got what they wanted. The mean is very close to 1, and the 95% CI for that mean does not dip below 0.9 (or a 90% response in the younger group compared to the older) on the low end. If we didn’t at this point know that neutralizing antibodies are a correlate of protection, I might be struggling to interpret this, but with that knowledge now at hand, I think it’s pretty clear that this vaccine has the desired effect in this younger age group.
You might have noticed here that the title on that table says patients “without evidence of SARS-CoV-2 infection up to 1 month after Dose 2.” Why would they want to use patients without evidence of SARS-CoV-2 infection? Well, because SARS-CoV-2 infection would boost antibody levels! Response to the virus would totally mess up the point of this experiment—measuring response to the vaccine. We want to know that the patients respond to the vaccine alone sufficiently to protect them, so anyone who had evidence of SARS-CoV-2 infection up to the measurement point was excluded to keep the results accurate for that question.
The briefing book also includes results for “response rate,” which was defined as a certain level of immune response to the SARS-CoV-2 reference strain (a US isolate4 named USA-WA1/2020, which is very similar to the original Wuhan lineage5), but these results add very little: there was no percentage difference in response rate between the two age groups. Just as many patients met the response criteria in one age group as the other: 99.2% of them, in fact.
Lastly, there were some vaccine efficacy data collected, but let’s remember these were not the primary endpoint of this trial and the trial was not designed to look at vaccine efficacy. There were only 19 total COVID-19 cases, which is a small number. Still, vaccine efficacy was estimated at 90.7% based on these events (95% CI: 67.4% to 98.3%) against confirmed COVID-19. While this result is based only on a small number of events, it is quite encouraging. Unfortunately, no variant sequencing information is available for the cases in question.
Coming to the question of variants, you will notice that the reference strain described above is not the Delta variant. It is not even the Alpha variant, which the Delta variant largely displaced in the US. How are we to contextualize the results seen here when Delta is the dominant variant in the current US epidemic? Thankfully, Pfizer did some exploratory work here, and looked at neutralizing titers against both the reference strain as well as the Delta variant in a separate experiment using samples from their patient population.
I’m about to show you those results, but I need to note that they will have different raw numbers from the table I showed earlier. This does not mean that the responses are lower. Immunological assays are generally very context-dependent in terms of the numbers they put out. Units are relative to the specific assay being conducted, and this next experiment was a different assay compared to what I showed earlier. What is important here is that we want to see a result where the Delta neutralizing response is similar (as in, not off by more than a factor of 10) compared to the reference strain neutralizing response, at the 1 month post-Dose 2 time point. We see that:

Admittedly, there is a really small number of patients being looked at in this experiment, but it looks good to me. The responses are similar against the two variants. That’s what we wanted to see and we saw it. Great!
Moving along, let’s discuss safety. The briefing book text reports that in the safety group (1511 patients in the vaccine group and 749 in the placebo group), up to 1 month after dose 2 administration, there were a total of 2 serious adverse events (SAEs), one in the vaccine group and one in the placebo group. Neither SAE was determined to be caused by study treatment, as evaluated both by the study committee and the FDA. There were no deaths in either group. A few more SAEs were reported in the full safety group out beyond the 1 month point, for a total of 4 in the vaccine group and 1 in the placebo group. None of the events were linked to treatment. One event in the vaccine group was a child who swallowed a penny, something that goes to show what an adventure pediatrics can be.
On to the less serious events, the most common reactions were pain at the injection site, fatigue, and headache. Most were mild reactions. You can review the details in the briefing book, but my overall feeling is that the vaccine looks pretty mild at this lower dosage in the 5-11 year old age group. We will need to continue to monitor its administration under the EUA, however, when that comes. I know that for several of you, and for myself as well, the very rare myocarditis events are of particular interest. That event is too rare to have been seen in a trial with only thousands of patients.
Overall, I think that reducing the dose clearly worked and Pfizer now has a version of its vaccine that works in the younger age group. A number of questions are about to flood in, such as:
The Moderna vaccine has had better and more durable responses than the Pfizer vaccine in adults (well, marginally better)—should I wait to vaccinate my child with the Moderna vaccine when it is approved?
If my child is 11 years and 11 months old at the day of EUA approval, should they get the lower dosage for both doses, the adult vaccine dose for both doses, or a mixture of the two?
If my child starts the Pfizer course but the Moderna vaccine is approved before they finish, can they get Moderna for their second dose?
The answer to these questions is to ask your child’s pediatrician. I have my personal thoughts—I don’t think mixing and matching vaccines is a good plan here, and I think it’s better not to wait if you have the chance to get a vaccine now vs later, but I also know the limits of my knowledge here. This is new science, and all decisions here should be made carefully. For the purposes of individual healthcare decisions, don’t believe anything you read about these vaccines without careful consideration and consultation with medical professionals. This includes things that you read in substack newsletters, no matter how trustworthy you think the author of each newsletter may be.
On my second question above, by the way, the answer is: I really don’t know. The cutoff for age groups had to lie somewhere. We know to expect favorable risk-benefit for a 12 year old given the adult dosage. I don’t know what to expect when it comes to using the child dose for the first inoculation and the adult dosage for the second. I do know that several children enrolled in the trial actually passed the age of 12 and were unblinded as to their vaccine-or-placebo status when they aged up, but I don’t think any turned 12 before getting the second dose (that would have been quite a failure of trial design if someone had been enrolled in that circumstance, really). I’m going to wait to see what I hear from prominent ID physicians before commenting on that, but I do think it’s a very interesting question.
One thing I do feel confident saying is that every child who is eligible for a vaccine should get the vaccine for which they are eligible, in consultation with their pediatrician, as soon as possible. So while the very small number of children who are 11 years old and 11 months at the date they get their first dose may be in a weird spot, I don’t think children who are 11 years old, 10 months, and 29 or 30 days old are in that same weird spot. Those children, if their healthcare providers agree, should get the child-sized dose because it is clearly very effective.
As for the VRBPAC meeting and next steps, the committee had some interesting things to say. One item that came up was concern that vaccination would become mandated for school attendance. Certain committee members were actually pretty concerned about this, because the vaccine will be under EUA initially. The committee felt that such mandates were unlikely until formal approval of the vaccine, and they have good reason to believe that—mandates have not been common in middle and high schools despite there being an existing EUA for children ages 12-15. Most schools and government authorities that run them appear to want to wait for full approval before requiring vaccination in a specific age group. The committee expected this trend to continue.
Now that VRBPAC has made its recommendations, the FDA will likely issue a formal EUA. Then, the CDC’s ACIP6 will weigh in, making the recommendations for how the vaccine should actually be used, and the CDC will issue final advice on the vaccine for this age group. The CDC’s final advice will be the last step, and the vaccine will then become available. I’ll continue to cover advancement in this story, as it happens.
What am I doing to cope with the pandemic? This:
Celebrating our daughter’s first month of life
On Thursday (tomorrow), our daughter will be one month old, and it’s amazing to see how she is starting to learn things about her world. People with experience of babies won’t be surprised by what I’m about to describe, but nonetheless putting it in words reminds me how amazing it all is.
A lot of the first four weeks of life are spent learning how your body works for the most basic tasks; eating, its inevitable outcomes, etc. The brain that a newborn comes out with changes rapidly in those first weeks, and starts to develop to be able to control voluntary functions like movement of arms, legs, and head. All of this development needs to happen after birth because of one really simple limiting factor in all mammalian birth: the pelvis. Having to fit through this narrow, relatively rigid bone limits the amount of brain development that can take place before birth.
The baby that is born on that first day has a brain almost like a flower bud. It has great potential for growth, but it’s not quite there yet. I’m enjoying watching the process of blooming.
You might have some questions or comments! Send them in. As several folks have figured out, you can also email me if you have a comment that you don’t want to share with the whole group.
Join the conversation, and what you say will impact what I talk about in the next issue.
Also, let me know any other thoughts you might have about the newsletter. I’d like to make sure you’re getting what you want out of this.
Part of science is identifying and correcting errors. If you find a mistake, please tell me about it.
Though I can’t correct the emailed version after it has been sent, I do update the online post of the newsletter every time a mistake is brought to my attention.
No corrections since last issue.
See you all next time. And don’t forget to share the newsletter if you liked it.
Always,
JS
The name changed for reasons that I am unqualified to comment on, not personally being Turkish.
Vaccines and Related Biologic Products Advisory Committee.
Because the trial was a “seamless” Phase 1/2/3 trial, with two Phase 2/3 cohorts enrolled, this date was different depending on which Phase the patient was enrolled for. Phase 1 patients had a cutoff date in mid-July, the first group of Phase 2/3 patients had a cutoff date in early September, and the second group of Phase 2/3 patients had a cutoff date in early October.
Sometimes I still hear misinformed/conspiracy nut folks claiming that SARS-CoV-2 has “never been isolated.” This is ridiculous. The virus has been isolated numerous times from infected patients, and this isolate is one example of that. You can read about its isolation in detail here: https://wwwnc.cdc.gov/eid/article/26/6/20-0516_article
For the biomedically inclined, you can read about this isolate from the BEI catalog here: https://www.beiresources.org/Catalog/animalviruses/NR-52281.aspx
American Committee on Immunization Practices.








Belated congrats on the birth of your daughter! Ours just turned 28 months. Parenting has been by far the most enriching experience of my life.
People just liked it better that way.