Greetings from an undisclosed location in my apartment. Welcome to COVID Transmissions.
It has been 921 days since the first documented human case of COVID-19. I have to skip the history fact today for length reasons.
Bolded terms are linked to the running newsletter glossary.
It has been quite some time since I last wrote, but I’ve bene eager to be able to write to you about the topics we’re going to cover today. Namely, we have some vaccine authorizations that deserve a deeper dive—the Novavax authorization, and the recommended authorization of the Pfizer and Moderna vaccines in children 6 months and up.
Keep COVID Transmissions growing by sharing it! Share the newsletter, not the virus. I rely on you to help spread good information, which you can do with this button:
Now, let’s talk COVID.
Novavax’s anti-COVID-19 vaccine product is recommended for authorization in the US
The VRBPAC1 of the US FDA has recommended that the protein subunit COVID-19 vaccine made by Novavax should be authorized for emergency use in the US.
This vaccine is very different from the mRNA vaccines or the Johnson and Johnson viral vector vaccine. It uses synthesized spike protein molecules to deliver its antigens and thus immunize patients against COVID-19. It is similar to the mRNA vaccines in that 2 doses are given initially, with a separation of 21 days.
The results of the studies that went into this briefing package have been known in a cursory, topline fashion for quite some time now—I covered them starting almost exactly one year ago, and then again in January:
The vaccine had excellent results back then, but it had a long road to approval. I mostly chalk this up to Novavax itself struggling to satisfy the regulatory requirements of FDA filings. I really cannot emphasize enough how difficult it can be to prepare a regulatory filing for the FDA. Having experienced regulatory affairs staff can be a make-or-break thing for a company. So it is rarely surprising to me when it takes a while for a company that has never marketed a product before to get its filing together correctly.
In fact, I’m more surprised that Moderna put theirs together so well and so quickly than I am that Novavax took a long time to get theirs completed.
Anyway, with the review of the data by the VRBPAC we get to see more detail, in the company’s own words, on what was seen in the trials for their vaccine. The briefing document can be found here: https://www.fda.gov/media/158912/download
Normally I would focus on the efficacy data here, but I think that would be somewhat pointless. These studies were conducted in a pre-Delta, pre-Omicron environment. At the time, this vaccine had results that looked as fantastic as the mRNA vaccines. I think the mRNA vaccines are still fantastic—they’ve saved a lot of lives and prevented a lot of serious disease—but since the Novavax vaccine was not tested against these new variants, all I can say is that I would expect it to perform similarly in the current environment. The exact numbers are hard to tease out without real-world data2 on this vaccine—which doesn’t exist since it hasn’t been used outside of clinical trials yet.
On the other hand, I understand the company has already learned from the experience of other vaccines and has started work on a booster dose regimen. Their technology would also allow them to create an omicron-specific vaccine with some amount of ease, though it might be a bit more challenging than for an mRNA technology from a process development perspective.
The delay has provided one particular advantage: safety follow-up. With more time, the company was able to follow patients for longer as far as safety events. The safety section of the briefing document is really good reading, and is more detailed than we saw for previous vaccines. It delves into rare adverse events of interest, including myocarditis.
We see in the analysis that there are rare myocarditis events associated with this vaccine. To me this is something of a surprise; I had thought that the myocarditis observation with mRNA vaccines had something to do with the vaccine technology, but this is a different technology—so I guess not! This bears more investigation.
The important thing here is that this event is rare, transient, and largely manageable. And it’s certainly no reason to avoid COVID-19 vaccination, given the much more clear and present dangers of that disease.
Speaking of avoidance, there are definitely still people out there who have been hesitant about COVID-19 vaccination because of the technologies used in available vaccines. Some have objected to the use of human cell lines in existing vaccines. Some are nervous about the perceived newness of mRNA technology.3 Some don’t like the Johnson and Johnson vaccine due to the blood clotting issue.
The Novavax option has none of these problems. It is made in insect cells, it uses protein-based technology, and does not have the viral vector that might have contributed to blood clotting. If you know someone who is still waiting because of concerns over technology, please try and prevail upon them to get this vaccine. You might be saving their life.
VRBPAC recommends authorizations of childhood vaccines
The biggest news today is from earlier this week, of course—that the Moderna and Pfizer vaccines were reviewed by the VRBPAC and they recommend that they be authorized in children 6 months old to 5 years of age. The votes for the Pfizer and Moderna vaccines were both unanimous, but there is more to the story than just that. As we’ll see in a moment.
This came right on the heels of a recommendation on Tuesday from the VRBPAC that Moderna’s vaccine be authorized in children 6-17 years of age. For that story, here is coverage from STAT news: https://www.statnews.com/2022/06/14/fda-panel-unanimously-backs-modernas-covid-vaccine-for-children-ages-6-to-17/
The first question that I see asked most frequently about these vaccines in the youngest children is this: why vaccinate? Isn’t COVID-19 less harmful in children?
I think this meme of lower severity is really insidious, and I want to emphasize that it largely began with an understanding of COVID-19 during a period when children were not in schools and exposed only to a limited extent. It also has some rhetorical flaws—for example, I could make a case that brain damage is less severe in children, but this is not a good reason to stop using batting helmets in Little League. When thinking about healthcare in children, you need to consider that even if a negative outcome is rare, given the early stage of the patient’s life, they are often stuck with the damage done for a very long time.
To get very specific, COVID-19 is a serious threat in children. Yes, many children don’t experience negative outcomes, but many also do experience them. Let me show you a couple of slides from yesterday’s meeting.
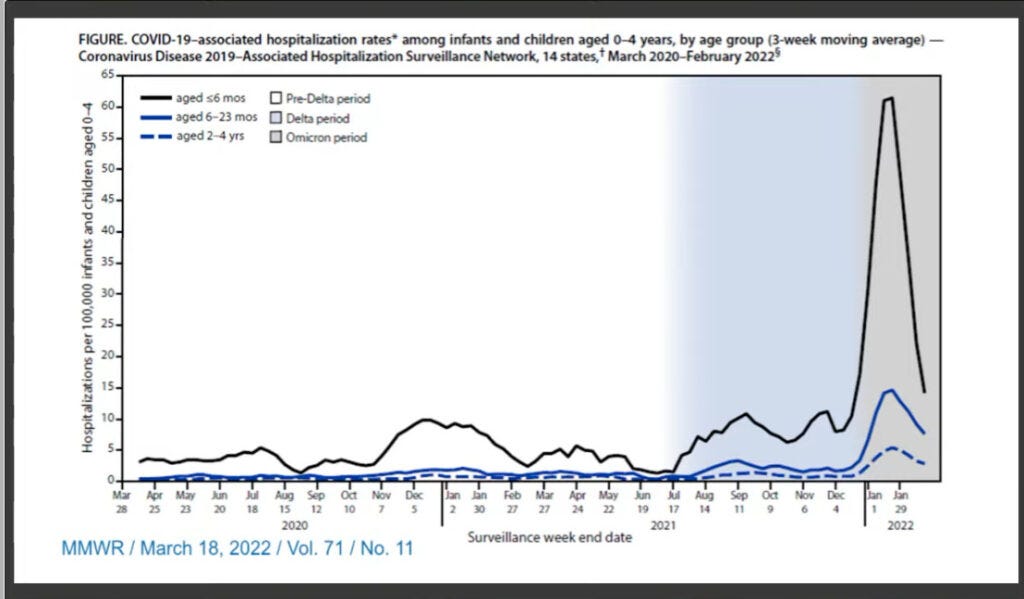
Here we can see that in an Omicron world, hospitalization rates among very young children rose alarmingly. And we know that since the virus is continuing to evolve to escape immunity, reinfections are possible and even previously-infected children may be vulnerable. A vaccine to reinforce children’s immunity could do a lot of prevent the most severe outcomes like hospitalizations.
Then there’s this, regarding deaths:

Compared to other viral diseases, there is no contest here. COVID-19 kills a lot of children. You don’t want your child to be one of them.
I think these slides on their own establish the unmet need for a pediatric COVID-19 vaccine, but I also want to emphasize that Long COVID exists and that vaccines may protect against it. Long COVID can happen to young kids too, though data are sparse. This is also a threat that you shouldn’t turn your back on.
The thing is, to match an unmet need, you need a vaccine that actually works. The numbers we saw yesterday suggest that both the Moderna and Pfizer vaccines will be effective in preventing disease in the youngest children. However, I don’t feel confident pinning those numbers down. the number of children who got three doses of the Pfizer vaccine was small, and that means there is a wide range of results. The raw number does not necessarily convey the true efficacy. Likewise, Moderna’s data were complicated by the Omicron wave, with parents sometimes being reluctant to bring their kids in for confirmatory PCR tests when they were sick.
With these issues in mind, it looks to me like vaccination reduces the risk of symptomatic disease by at least 50 percent with either vaccine. Cross-comparing these trials is not appropriate, but I am going to mention that Pfizer required 3 doses to see an effect, meaning it takes longer to get to an effective dose, and Moderna did not. It is better to be vaccinated sooner rather than later.
Additionally, the Moderna vaccine is already having a booster developed, and that booster is supposed to be Omicron-specific. Pfizer is not Omicron-specific, yet.
For this one, I think I’m leaning Moderna, but for my child, I’m going to take the vaccine that is offered. Holding out for the choice you perceive as ideal is a mistake.
On the safety front, there were some instances of fever and loss of appetite reported. It is hard to solicit adverse events in this age group, but these sorts of patterns are pretty typical for pediatric vaccines. Nothing jumps out at me here as a serious concern.
The bottom line is, we have two vaccines here that can fill the unmet need with solid tolerability. The vote to authorize them was unanimous on both counts, and if the FDA ultimately makes these vaccines available, we have tools to blunt the severity of COVID-19 in all age groups. Vaccination during pregnancy can protect infants for the first 6 months of life, followed by vaccination after that point. A person of any age will be able to be protected from COVID-19—even someone too young to wear a mask. I can’t emphasize enough the importance of this milestone.
I hope the FDA moves quickly to authorization now that this recommendation has been issued.
You can read everything that was presented today here, and see the evidence for yourself: https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-june-14-15-2022-meeting-announcement#event-materials
Part of science is identifying and correcting errors. If you find a mistake, please tell me about it.
Though I can’t correct the emailed version after it has been sent, I do update the online post of the newsletter every time a mistake is brought to my attention.
No corrections since last issue.
What am I doing to cope with the pandemic? This:
Stir Fry Experiments
I’ve been playing around with different ways to stir-fry chicken lately. This experiment involved a cornstarch coating (a light one), after marinating in a soy-gochujang-honey mixture. I added some brussels sprouts too, which took well to those flavors.
I can’t quote the comments today because I’m out of email length room. But do go have a look at last issue’s comments!
You might have some questions or comments! Join the conversation, and what you say will impact what I talk about in the next issue. You can also email me if you have a comment that you don’t want to share with the whole group, or if you are unable to comment due to a paywall.
If you liked today’s issue, please consider becoming a paid subscriber and/or sharing this newsletter with everyone you know.
Please know that I deeply appreciate having you as readers, and I’m very glad that if we must be on this pandemic journey, at least we’re on it together.
Always,
JS
Vaccines and Related Biologic Products Advisory Committee, a standing group of experts not employed by the FDA nor by the pharmaceutical companies, who advise the FDA.
“Real-world data” is used as a term to refer to medical studies conducted outside of clinical trials. I promise you that clinical trials also happen in the real world, but since they are structured experiments they don’t closely resemble typical patient care.
I must emphasize that mRNA technology is not really new. It has been in development for decades with many successful human studies.










Hi John, Well, a family member has tested positive but the rest of us are still negative. We are doing the best we can to isolate within our abode. We are doing our best to maximize ventilation. My question: What is the latest/best on what temperature or relative humidity minimizes transmission? We can set both of these on our thermostat. Thanks!
John, you write that Nuvaxovid, "... hasn’t been used outside of clinical trials yet." That is not 100% accurate. Millions of doses have been given in other nations, e. g. Australia. Just not here in the USA.
Forbes reports https://www.forbes.com/sites/alexknapp/2022/02/28/novavax-highlights-enduring-covid-immune-response-six-months-after-vaccination/) that Nuvaxovid has relatively long-lasting immunity, fading more slowly than mRNA vaccines (at least according to Novavax's own data).